Warning: Undefined variable $page_cta in /var/www/vhosts/dev.fastspot.com/httpdocs/clients/siena/templates/basic/content.php on line 10
SPRING 2023 IN-PERSON RESEARCH POLICY
Initially posted 9/26/2022 (Provost-Approved Policy)
In-person research must follow Siena’s Marching Forward Plan and CDC Recommendations at the time of data collection. Currently, the CDC recommends indoor masking when the community transmission level (Albany County for Siena College) is "high". Both policies are fluid/subject to change.
On-Campus Research
Exceptions for faculty/staff research protocols are as follows:
- Exception #1: Vaccinated participants may be unmasked for an essential study procedure if the procedure cannot be performed with a mask, but study staff must always be masked. Examples of essential study procedures: required eating or drinking, use of a vection drum to induce nausea, measurement of respiratory functions (e.g., use of a spirometer).
- Exception #2 (Applies to Off-Campus Research): Participants who:
· Are under the age of 2
· Have a medical condition, mental health condition, developmental or cognitive condition, or disability that makes it hard for them to wear a mask
· Are deaf and move their face and mouth to communicate
· Have trouble breathing, are unconscious, or are unable to remove a face covering without help
These are the minimal requirements.
Off-Campus Research
All researchers will be expected to adhere to Siena’s IRB safety protocols when conducting research at off-campus locations. If the off-campus location has more stringent protocols in place, then those must be followed while at the location.
The PI's description of the safety plan and/or exemption request must be on file with and approved by the IRB before data collection begins. This can be done as part of the initial application process or via email to irb@siena.edu.
**********************************************
POLICY CHANGE working with individuals from vulnerable populations
Effective 10/1/19, all PIs (and their supervisors) who are recruiting individuals from vulnerable populations must provide verification of additional CITI training. Vulnerable populations (e.g., children, prisoners, individuals with impaired decision-making capacity, terminally ill patients, institutionalized individuals, people who are socially or economically disadvantaged) require additional considerations and protections. The guidelines are not thoroughly covered in the basic CITI training required for all PIs. More information can be found here as well as under "Online Certification Training."
**********************************************
CHANGES TO THE COMMON RULE (JAN 2019):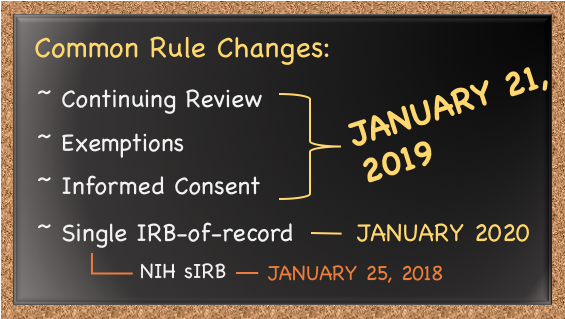
There were major changes to the Common Rule in January, 2019 which affect all researchers at Siena. Generally the changes involve reducing administrative burden, with less risky studies requiring less paperwork, and better protecting participants by making informed consent more meaningful.
Highlights:
- The number of exempt categories increased from 6 to 8, and several allow for minimal risk and collection of personally identifiable information. (Research that may have been categorized as expedited before break may now be exempt.)
- Most expedited research (in addition to exempt) no longer requires continuing review (renewal)
- You don't need a consent form for exempt studies that are no risk
- Expedited studies are automatically categorized as minimal risk
- Pregnant women are no longer considered "vulnerable" populations (!)
This doesn't affect research approved prior to January 21, 2019.
Before submitting your next application, you are encouraged to carefully review the "IRB Application Categories" and changes to the consent process. The online application has been updated to reflect the new federal policy. "Changes to the Common Rule" offers a more detailed explanation of what's new and different.
Please reach out to irb@siena.edu with questions! A series of IRB trainings to assist you in the application process will be offered throughout the semester and announced on the Digest. If you are teaching a class in which several students will be submitting an application, the IRB welcomes the opportunity to come to your class.
THE IRB AND HUMAN SUBJECTS RESEARCH
Per federal regulations as prescribed in 45 CFR Part 46, any faculty, student and/or administrator/staff planning to engage in research involving human subjects must receive approval from the Siena College Institutional Review Board (IRB) prior to initiating any research activities, on or off campus.
What constitutes research or a research project? The US Office for Human Research Protections (OHRP) defines research as any “systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”
What is a human subject? It seems obvious but there is a OHRP definition for this as well. A human subject is a living individual about whom an investigator conducting research obtains (1) data through intervention or interaction with the individual, or (2) Identifiable private information.
ROLE OF THE SIENA COLLEGE IRB
The Siena College IRB provides oversight of OHRP guidelines. These guidelines help to assure that the wellbeing and dignity of individuals involved in research conducted at Siena College or by Siena College constituents are protected.
The IRB evaluates (1) the role of assessment of risk-benefit criteria in the determination of the appropriateness of research involving human subjects, (2) appropriate guidelines for the selection of human subjects for participation in such research, and (3) nature and definition of informed consent in various research settings.
Related Reports:
- Belmont Report (summary)
- Nuremberg Code
- WMA Declaration of Helsinki
- Human Experimentation: The Good, Bad & the Ugly (SciShow YouTube Video on historical perspective of above reports)
REVIEW TYPES
The IRB employs four (4) primary types of review depending on the degree of risk to human subject(s):
Exempt Review: Feedback sent 1-2 weeks after IRB application is submitted.
Exempt Limited Review: Feedback sent 2-3 weeks after IRB application is submitted.
Expedited Review: Feedback sent 2-3 weeks after IRB application is submitted.
Full Review: Full committee review conducted 1-3 months after IRB application is submitted.
Please see "IRB Application Categories" for a full description of each type of review.
REGULATIONS & RESOURCES
Regulations
- Protection of Human Subjects, 45 CFR 46
- Family Educational Rights and Privacy Act (FERPA)
- Siena College Research Misconduct Policy
Resources